Introduction:
Splenic marginal zone lymphoma (SMZL) is a rare, indolent disease. There is no universally accepted standard therapy and long-term outcome data are limited. Rituximab monotherapy is increasingly used over splenectomy in the first-line setting despite a paucity of comparative studies. In the absence of randomized trials, large-scale international real-world data studies are critical to evaluate outcomes of different treatment interventions and to understand the natural history of SMZL in the rituximab-era.
Methods:
Adult patients with SMZL diagnosed between Jan 1 st 2000 and Dec 31 st 2018 were identified using regional/nationwide population-based registries or hospital registries in Europe, the UK, North America, and Australia. Medical records and pathology reports from eligible patients were reviewed locally by clinicians with specialty experience in hemato-oncology. SMZL cases were required to meet the diagnostic criteria by Matutes et al. Detailed data on clinico-pathological features, comorbidities, treatments (including treatment intention), treatment responses and survival outcomes were entered in a central study database. Overall survival (OS) was defined as time from diagnosis until death from any cause or censoring at last follow-up unless stated otherwise. Event-free survival (EFS) was defined as the time from diagnosis until histological transformation, initiation of new chemotherapy or death from any cause. Survival probabilities were estimated using the Kaplan-Meier estimator. Cumulative risk of transformation was estimated with death as a competing risk. Primary endpoints were 10-year OS and 5-year EFS respectivly.
Results:
A total of 934 patients from 25 sites were included. Median age at diagnosis was 68 years (IQR 60-76) and 54% were female. Bone marrow involvement was present in 96% (564/590) of those assessed, anemia (Hb <12g/dL for women or <13g/dL for men) in 58%, LDH >UNL in 46%, lymphocytosis (>5 x10 9/L) in 34%, and B-symptoms in 34%. Treatment data were available for 97% of patients. Median follow-up was 9.1 years (range 0-23). Watch and wait was the initial strategy in 28% and 88% of patients received at least one line of treatment during follow-up with the most common being splenectomy (45%), immuno-chemotherapy (19%) or rituximab monotherapy (8%). Fifteen percent received “other” treatments (i.e., combinations of listed therapies and/or radiation). The overall response rate to first-line treatment was 88% (95%CI: 85-90%) with 61% (95%CI: 57-64%) achieving CR. Second line (2L) therapy was needed in 34% during follow-up, 3L in 20% and 4L+ in 9%.
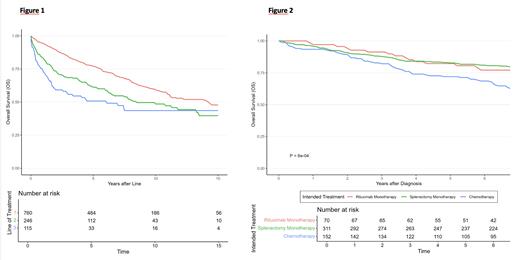
The 5-year EFS and OS for all patients were 61% (95%CI: 57-65%) and 77% (95%CI: 74-80%) respectively. The 10-year OS and lymphoma-specific mortality (with non-SMZL deaths as competing risk) estimates were 60% (95%CI: 57-64%) and 11% (95%CI: 9-14%) respectively. The 5-year OS after 2L and 3L were 61% (95%CI: 55-68%) and 51% (95%CI: 42-61%) respectively (figure 1). The 10-year cumulative risk of histological transformation was 17% (95%CI: 13-21%), with 5-year post-transformation OS of 42% (32-54%). At the time of transformation, 60% were chemo-naive and 52% had never been treated for SMZL. The 5-year OS for chemo-naive patients with transformation was 54% (95%CI: 41-71%) versus 24% (95% CI: 13-44%) for transformations after (immuno)chemotherapy (p = 0.01).
When comparing patients intended for treatment in first line with splenectomy (n=312) versus rituximab (n=70), patients treated with splenectomy more frequently presented with extra-hilar lymphadenopathy (35% vs 18%), splenomegaly (92% vs 87%) and anemia (64% vs 48%). OS was similar for the two groups (Figure 2, p = 0.53). The 5-year EFS were 63% (95%CI: 57-68%) and 64% (95%CI: 54-77%), respectively (p = 0.31).
Conclusions:
To date, this is the largest real-world study of SMZL with detailed treatment and outcome data. The study shows that histological transformation was not uncommon with 10-year risk of 17%. However, outcomes for the entire cohort were excellent irrespective of initial treatment and both OS and EFS were similar after first line rituximab versus splenectomy. Long-term survival remained relatively high even following multiple lines of therapy.
Disclosures
Cheah:Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Genmab: Consultancy, Honoraria; TG therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BeiGene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Menarini: Consultancy, Honoraria; AbbVie: Research Funding; MSD: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: TRAVEL, ACCOMMODATIONS, EXPENSES, Research Funding; Lilly: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZenecca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Ascentage Pharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Daizai: Consultancy, Honoraria. Cerhan:NanoString: Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genmab: Research Funding; Protagonist: Other: Safety Monitoring Committee; Genentech: Research Funding. Clausen:Incyte: Consultancy; Genmab: Consultancy, Other: advisory; Roche: Other: Travel funding; Aztrazenica: Other: advisory; Gilead: Consultancy; Janssen: Consultancy, Other: advisory; Abbvie: Consultancy, Other: advisory. Eyre:Loxo Lilly: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Autolus: Consultancy; PeerView: Speakers Bureau; Medscape: Speakers Bureau; KITE: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Eli Lilly and Company: Consultancy, Honoraria, Speakers Bureau; Secura Bio: Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Speakers Bureau; Loxo Oncology: Consultancy, Honoraria, Other, Speakers Bureau; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Beigene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Ghesquieres:Gilead, Roche: Consultancy; Gilead, Roche, BMS, Abbvie: Honoraria. Habermann:Genentech: Research Funding; BMS: Research Funding; sorrento: Research Funding. Hawkes:Gilead: Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses, Research Funding, Speakers Bureau; Merck KgA: Research Funding; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding; Regeneron: Speakers Bureau; Specialised Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Antengene: Membership on an entity's Board of Directors or advisory committees; Beigene: Other; Merck Sharpe & Dohme: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Other; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding. Opat:Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astra Zeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Beigene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck Sharpe & Dohme: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Honoraria, Research Funding; Roche: Honoraria, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees; CSL Behring: Membership on an entity's Board of Directors or advisory committees; Antengene: Membership on an entity's Board of Directors or advisory committees. Ekstroem Smedby:AbbVie: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Janssen: Research Funding. Trotman:BeiGene: Research Funding; Roche: Research Funding; Janssen: Research Funding; BMS: Research Funding; Cellectar: Research Funding. Villa:Roche, AstraZeneca, Abbvie, Janssen, Kite/Gilead, BMS/Celgene, BeiGene, Merck: Consultancy, Honoraria; Roche, AstraZeneca: Research Funding.